The U.S. lead pipe catastrophe
This weeks news in the U.S press has been dominated by news from Flint, Mitchigan, where its population of 100,000 has been drinking contaminated tap water. The chief contaminants were found to be lead, pathogenic bacteria and trihalomethanes. The crisis came to head on Saturday January 16th when President Obama signed a federal state of emergency. He has now allocated $80 million to the city. The Singer Cher later announced that she would be donating 181,440 bottles of spring water from Iceland to the people of the city saying that the Flint water crisis “was a tragedy of staggering proportions….”. The presidential candidate Hillary Clinton has since spoken out and said she was “outraged by what had happened”.
For the last 40 years the city of Flint had been buying treated tap water from the publically owned Detroit Water and Sewage Department (DWSD). This water was pumped to Flint over a distance of 69 miles. Then in May 2014 Flint started to treat its own water taken from the River Flint. This was taken as a temporary measure whilst a new pipeline was built from Lake Heron to Flint. Changing water isn’t unusual for the water industry but doing so without properly evaluating the chemistry of the new water on the pipe infrastructure is extraordinary.
To understand what happened you have to compare the differences between two types of water differ and how the differences effect the concentrations of the contaminants.
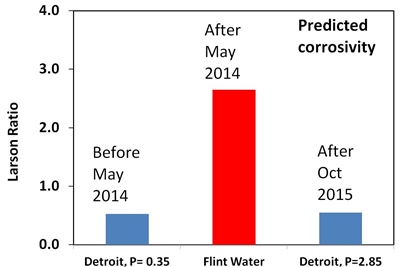
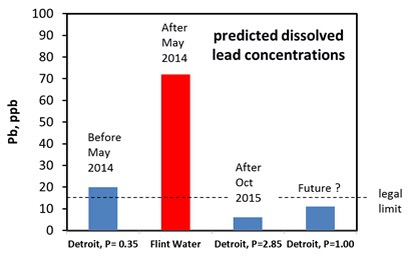
Lead contamination comes chiefly from the lead water pipes that connect homes to the mains water pipe in the street. These pipes were used up to about 1970. Lead was relatively cheap, could be easily bent, corrosion resistant and pressure resistant. Ideal for pipes except that lead is harmful to health. There has been a lot of research on lead release from water pipes and we now know that the temperature, pH, alkalinity (the type and quantity of carbonate present), the solubility of lead minerals and the length of lead pipe are important factors that control the concentration of dissolved lead. We also know that phosphate helps to reduce the concentration of lead and that chloride may increase it. Ideally you want very insoluble minerals to grow on the inner walls of the pipe as the lead will become part of the mineral layer rather than accumulate in the water. You can predict the maximum concentration of dissolved lead using software written by the U.S. Geological Survey. The graph shows what happened when Detroit Water was switched to River Flint Water.
The difference is that phosphate was added to the Detroit Water but not to the treated Flint River water. Both Detroit Water and the treated Flint River Water have an average pH of 7.4. This is an ideal pH but only if phosphate is added to the tap water. Its not a good pH if phosphate is absent. It is interesting that Detroit Water only contained 0.35mg/L phosphate (P), which still puts the predicted concentration of lead to be above the legal limit of 15 ppb. Why didn’t Detroit Water use the recommended amount of 1.0mg/L? Currently, the amount of phosphate added to Detroit water is 0.35mg/L plus an extra 2.85mg/L added by Flint Water. The decision to go well above 1.0 mg/L might be a precautionary measure but it may have to come down to 1.0mg.L in the future.
Certain chemicals help to speed up the rate at which a metal is attacked or corroded and one of these is chloride. Metals such as iron and steels corrode particularly well in the presence of high concentrations of chloride. The treated Flint Water contained up to eight times as much chloride as the Detroit Water making it more corrosive to the cast iron water pipes in the distribution system. You can predict how corrosive a tap water will be by calculating the Larson Ratio, which is the ratio of chloride and sulphate to bicarbonate, Detroit Water has a Larson Ratio of 0.5, which is acceptable, whereas treated Flint River water has a Larson ratio of 2.6. Anything above 1.5 is regarded as being high and would be expected to greatly increase the rate of corrosion.
Cast iron pipes, like lead pipes, have their own mineral layer made of orange coloured iron oxides. The high chloride concentration would have destabilised this layer releasing iron oxide particles into the water. Whilst some orange water is to be expected from time to time a persistent amount of orange water should have been taken as evidence that something was seriously wrong within the water distribution network.
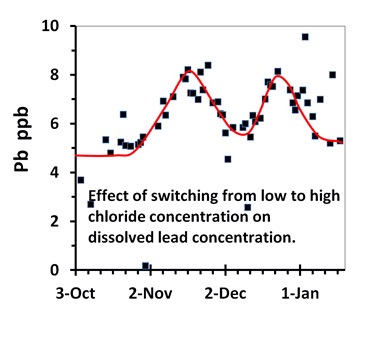
Chloride also appears to enhance the corrosion of lead. This is a relatively recent finding by Professor Marc Edwards team. The graph shows what happens to the concentration of lead when tap water is twice switched from low to high chloride concentration (U.K. study). The left shoulder of each peak corresponds to the high chloride concentration. The increase of 3 ppm may seem small but this graph is for phosphate treated tap water. A larger increase would have been observed if no phosphate had been used.
There are other issues at play. The treated Flints River water should have reached a maximum concentration of 80 ppm. Yet some of the reported lead concentrations have been much higher than this. This can only be explained by the presence of particulate lead, which are solid particles of lead containing material dispersed in the tap water. There are two likely sources, one is that the particles were fragments of the mineral scale that had become detached from the walls of the pipe. This is the usual explanation that is given. The other possibility is that dissolved lead accumulated on the orange iron oxide particles as they travelled down the lead pipe. Iron oxide particles tend to stick together and to the walls of lead pipes but will detach when the tap is turned on.
It is hard to work out what precisely happened at Flint without access to all the information. Water chemistry isn’t an easy subject and there are many of variable. It does seem that the operators of the Flint Water treatment works really struggled to understand what was happening. At first there were problems with odour. Then they found that they couldn’t maintain the chlorine disinfectant levels throughout the pipe with some regions using up chlorine much faster than others. Flint responded to the disinfectant issue by increasing the concentration of chlorine but this increased the amount of trihalomethanes to above acceptable limits. They also repeatedly flushed the network to prevent stagnant regions from forming. Then there were problems with colour. It appears that Flint responded by adding more ferric chloride flocculent, which removes organics. But this doubled the concentration of chloride from 45 mg/L to 85mg/L. There were leaks and an incident when sewage entered the system. They never added phosphate to mitigate for lead and they never evaluated how corrosive the River Flint water would be. It seems incredible that you would completely switch from one water source to another without understanding the chemistry and putting your customers at risk.
The chemical reasons for the catastrophe can be summarised as:
- the use of Flint River water of high chloride content
- the decision to adjust the water to pH 7.4 without adding phosphate
- the use of Detroit Water with only 0.35mg/L phosphate prior to using Flint water. It’s likely that the lead problem existed before 2014.
——————————————————————–
More on Lead in tap water
1. For an animation see https://jezhud.wordpress.com/2016/02/02/lead-pipe-animations/.
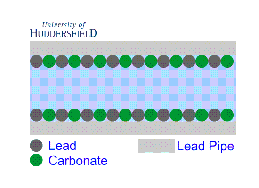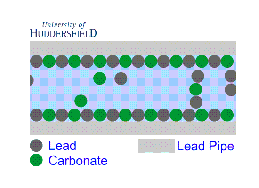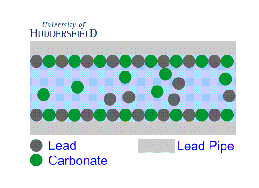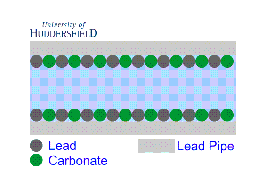
2. For images of the lead mineral scale


Dear Sir,
Thank you for a clear explanation.
It is reported that Flint used lime to control water hardness & corrosion, can you please comment on the chemistry of this ?
http://michiganradio.org/post/flint-had-no-plan-minimize-lead-corrosion-peoples-drinking-water-post-river-switch
LikeLike
Lime is calcium hydroxide is commonly used at water treatment works to raise the pH. During the water treatment process flocculants are added to remove particulate matter and organics from the water. This also removes bacteria. These flocculants are acidic and will reduce the pH to about 3.0. Ferric chloride was used by Flint. After flocculation, Bases such as lime are added to bring the pH back up. It’s up to the water company what pH they raise it too. If phosphate is added then its usual to bring the pH up to about 7.4. Hope this helps.
LikeLike
Thanks very much for this article.
Try as I might, until discovering this post I have been unable – as a lay person with a couple of semesters of undergraduate chemistry thirty years ago – to find on the internet any sort of précis account of the actual chemistry involved with this incident, even at the Virginia Tech website.
LikeLike
Thanks Robert. I work on lead in tap water for water utilities in the U.K. The water companies operate differently to the U.S. But the chemistry is still the same. I will try to put another post out in a couple of weeks time. Have a look at the animation that we put together it’s also on ten WordPress site.
LikeLike